Michael S. Cowled, Hang Li, Cameron L. M. Gilchrist, Ernest Lacey, Yit-Heng Chooi, and Andrew M. Piggott
Journal of Natural 86, 3, 541-549.
Publication Date: December 16, 2022
https://doi.org/10.1021/acs.jnatprod.2c00946
Abstract:
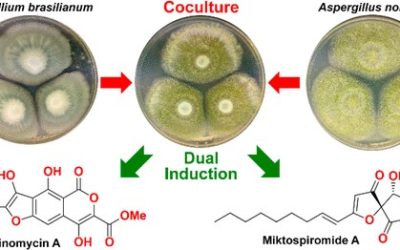
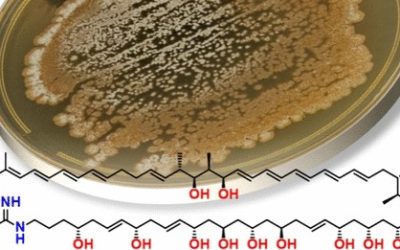
Penicillium turbatum has previously been reported to produce A26771B, a 16-membered macrocyclic polyketide with activity against Gram-positive bacteria, mycoplasma, and fungi, as well as the structurally related compounds berkeleylactone E and berkeleylactones I–O. In this work, large-scale cultivation of P. turbatum NRRL 5630 on rice yielded seven new berkeleylactone analogues, berkeleylactone E methyl ester, 14-epi-berkeleylactone F, berkeleylactones P–R, 12-epi-berkeleylactone Q, and 13-epi-berkeleylactone R, and six previously reported analogues, A26771B and berkeleylactones E–G and J–K. The structures of the berkeleylactones were elucidated by detailed analysis of spectroscopic data, molecular modeling, and comparison with literature values. Interestingly, six of the berkeleylactone analogues were isolated as pairs of hydroxy epimers, highlighting how Nature can exploit stereodivergence in biosynthetic pathways to increase chemical diversity. The genome of P. turbatum was sequenced, and a putative gene cluster (bekl) responsible for the biosynthesis of the berkeleylactones was identified. The new berkeleylactone analogues exhibited no significant biological activity against a panel of bacteria, fungi, the parasite Giardia duodenalis, or NS-1 murine myeloma cells, suggesting a hitherto undiscovered biological role.