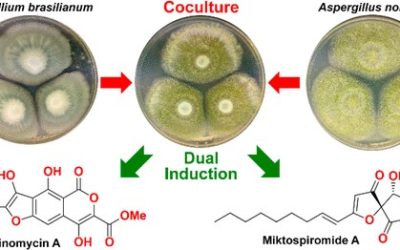
Robert J. Capon *, Ranjala Ratnayake, Michael Stewart, Ernest Lacey, Shaun Tennant and Jennifer H. Gill
Organic and Biomolecular Chemistry, 3: 123-129.
Publication Date: October 28, 2004
https://doi.org/10.1039/B413440K
Abstract:
Biological and chemical profiling of an Australian strain of the fungus Aspergillus unilateralis (MST-F8675), isolated from a soil sample collected near Mount Isa, Queensland, revealed a complex array of metabolites displaying broad chemotherapeutic properties. Noteworthy among these metabolites were a unique series of highly modified dipeptides aspergillazines A–E, incorporating a selection of unprecedented and yet biosynthetically related heterocyclic systems. Co-occurring with the aspergillazines was the recently described marine-derived fungal metabolite trichodermamide A (cf. penicillazine), whereas re-fermentation of A. unilateralis in NaCl (1%) enriched media resulted in co-production of the only other known example of this structure class, the marine-derived fungal metabolite trichodermamide B. Further investigation of A. unilateralis returned the known terrestrial fungal metabolite viridicatumtoxin as the cytotoxic and antibacterial principle, together with E-2-decenedioic acid, ferulic acid, (7E,7′E)-5,5′-diferulic acid and (7E,7′E)-8,5′-diferulic acid. The aromatic diacids have previously been reported from the chemical and enzymatic (esterase) treatment of plant cell wall material, with their isolation from A. unilateralis being their first apparent reported occurrence as natural products. Structures for all metabolites were determined by detailed spectroscopic analysis and, where appropriate, comparison to literature data and/or authentic samples.