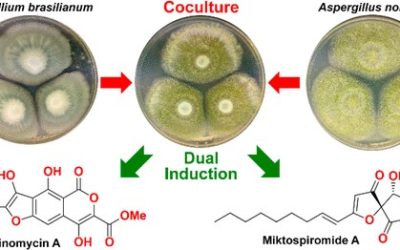
Hang Li, Cameron L. M. Gilchrist, Chin-Soon Phan, Heather J. Lacey, Daniel Vuong, Stephen A. Moggach, Ernest Lacey, Andrew M. Piggott, and Yit-Heng Chooi
J. Am. Chem. Soc., 142, 7145-7152.
Publication Date: March 17, 2020
https://doi.org/10.1021/jacs.0c01605
Abstract:
1-Benzazepine is a pharmaceutically important scaffold but is rare among natural products. Nanangelenin A (1), containing an unprecedented 3,4-dihydro-1-benzazepine-2,5-dione-N-prenyl-N-acetoxy-anthranilamide scaffold, was isolated from a novel species of Australian fungus, Aspergillus nanangensis. Genomic and retrobiosynthetic analyses identified a putative nonribosomal peptide synthetase (NRPS) gene cluster (nan). The detailed biosynthetic pathway to 1 was established by heterologous pathway reconstitution in A. nidulans, which led to biosynthesis of intermediates nanagelenin B–F (2–5 and 7). We demonstrated that the NRPS NanA incorporates anthranilic acid (Ant) and l-kynurenine (l-Kyn), which is supplied by a dedicated indoleamine-2,3-dioxygenase NanC encoded in the gene cluster. Using heterologous in vivo assays and mutagenesis, we demonstrated that the C-terminal condensation (CT) and thiolation (T3) domains of NanA are responsible for the regioselective cyclization of the tethered Ant-l-Kyn dipeptide to form the unusual benzazepine scaffold in 1. We also showed that NanA-CT catalyzes the regioselective cyclization of a surrogate synthetic substrate, Ant-l-Kyn-N-acetylcysteamine, to give the benzazepine scaffold, while spontaneous cyclization of the dipeptide yielded the alternative kinetically favored benzodiazepine scaffold. The discovery of 1 and the characterization of NanA have expanded the chemical and functional diversities of fungal NRPSs.