Ben Clark, Robert J. Capon, Michael Stewart, Ernest Lacey, Shaun Tennant, and Jennifer H. Gill
Journal of Natural Products, 67: 1729-1731.
Publication Date: September 1, 2004
https://doi.org/10.1021/np049826v
Abstract:
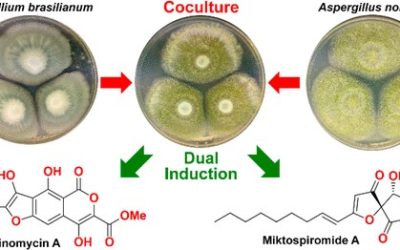
Chemical analysis of an Australian Streptomyces species yielded a range of known anthracyclines and biosynthetically related metabolites, including daunomycin (1), ε-rhodomycinone (2), 11-hydroxyauramycinone (3), 11-hydroxysulfurmycinone (4), aklavinone (5), bisanhydro-γ-rhodomycinone (6), and the anthraquinone 7, as well as the hitherto unreported blanchaquinone (8). The structure assigned to 8 was secured by detailed spectroscopic analysis and correlation to known analogues, such as the anthraquinone 7. This account also represents the first natural occurrence of 3, 4, and 7 and the first spectroscopic characterization of 11-hydroxysulfurmycinone (4).