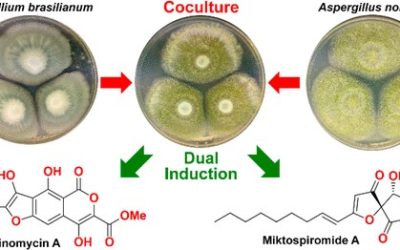
Jinyu Hu, Farzaneh Sarrami, Hang Li, Guozhi Zhang, Keith A. Stubbs, Ernest Lacey, Scott G. Stewart, Amir Karton, Andrew M. Piggott and Yit-Heng Chooi
Chem. Sci., 2019, 10, 1457-1465.
Publication Date: November 22, 2018
Abstract:
Perylenequinones are a class of aromatic polyketides characterised by a highly conjugated pentacyclic core, which confers them with potent light-induced bioactivities and unique photophysical properties. Despite the biosynthetic gene clusters for the perylenequinones elsinochrome A (1), cercosporin (4) and hypocrellin A (6) being recently identified, key biosynthetic aspects remain elusive. Here, we first expressed the intact elc gene cluster encoding 1 from the wheat pathogen Parastagonospora nodorum heterologously in Aspergillus nidulans on a yeast-fungal artificial chromosome (YFAC). This led to the identification of a novel flavin-dependent monooxygenase, ElcH, responsible for oxidative enolate coupling of a perylenequinone intermediate to the hexacyclic dihydrobenzo(ghi)perylenequinone in 1. In the absence of ElcH, the perylenequione intermediate formed a hexacyclic cyclohepta(ghi)perylenequinone system via an intramolecular aldol reaction resulting in 6 and a novel hypocrellin 12 with opposite helicity to 1. Theoretical calculations supported that 6 and 12 resulted from atropisomerisation upon formation of the 7-membered ring. Using a bottom-up pathway reconstruction approach on a tripartite YFAC system developed in this study, we uncovered that both a berberine bridge enzyme-like oxidase ElcE and a laccase-like multicopper oxidase ElcG are involved in the double coupling of two naphthol intermediates to form the perylenequinone core. Gene swapping with the homologs from the biosynthetic pathway of 4 showed that cognate pairing of the two classes of oxidases is required for the formation of the perylenequinone core, suggesting the involvement of protein–protein interactions.