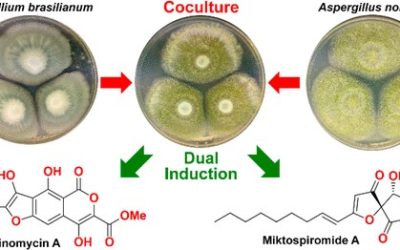
Michelle Farrugia, Nicholas Trotter, Soumini Vijayasarathy, Angela A. Salim, Zeinab G. Khalil, Ernest Lacey, Robert J. Capon
Tetrahedron Letters 2014, 55, 5902-5904.
Publication Date: August 28, 2014
https://doi.org/10.1016/j.tetlet.2014.08.116
Abstract:
Chemical fractionation of the southern Australian marine sponge Phoriospongia sp. (CMB-03107) yielded phorioadenine A (1) as a nematocidal agent and the first reported example of a 6-N-acyladenine natural product. The structure of 1 was confirmed by spectroscopic analysis and the chemical synthesis of racemic (1a) and enantiomeric (1b) analogues. HPLC–ESIMS analysis of the crude sponge extract with comparisons to the synthetic 6-N-acyladenosine 2a provided evidence that the biosynthetically related adenosine, phorioadenosine A (2), was present as a trace co-metabolite. The rare starfish metabolite asterubine (3) was also isolated as a co-metabolite, and its structure confirmed by spectroscopic analysis and chemical synthesis. Biological investigations confirmed that natural products 1–3 and synthetic analogues 1a–e and 2a were not cytotoxic to multiple mammalian cancer cell lines, or Gram-positive or -negative bacteria. Nematocidal activity (inhibition of larval development of Haemonchus contortus) detected in the Phoriospongia sp. extract was attributed to 1 (LD99 31 μg/mL), with preliminary structure–activity relationship investigations confirming the importance of the N-acyl side chain.