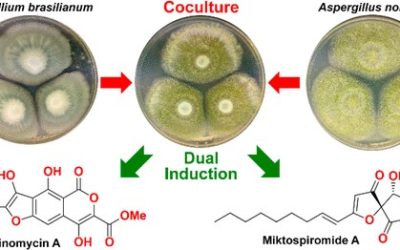
Alexander F. Moore, Heather J. Lacey, Andrew Crombie, Ernest Lacey, Andrew M. Piggott
Tet. Letters 2016, 57, 4224–4227.
Publication Date: August 20, 2016
https://doi.org/10.1016/j.tetlet.2016.08.014
Abstract:
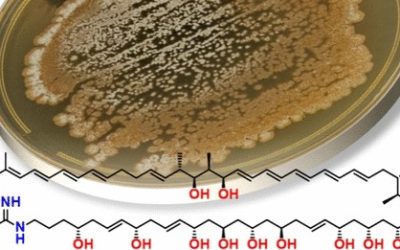
Herein, we report the preparation and characterisation of the primary pH degradation products of doramectin, a potent second-generation endectocide. Under mildly acidic conditions, doramectin underwent sequential deglycosylation to yield doramectin monosaccharide and doramectin aglycone respectively. Under basic conditions, doramectin isomerised reversibly to give 2-epidoramectin and irreversibly to give Δ2,3-doramectin. The isomerisation of doramectin to Δ2,3-doramectin proceeded stereoselectively, exclusively yielding the 4S epimer.