Leith Fremlin, Michelle Farrugia, Andrew M. Piggott, Zeinab Khalil, Ernest Lacey and Robert J. Capon
Organic Biomolecular Chemistry Chem. 2011, 9, 1201-11.
Publication Date: October 29, 2010
https://doi.org/10.1039/C0OB00654H
Abstract:
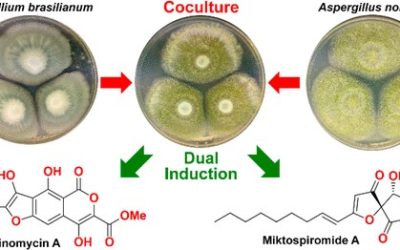
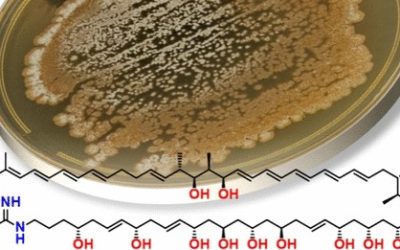
Chemical analysis of fermentation products from two Australian Streptomyces isolates yielded all four known and twelve new examples of the rare reveromycin class of polyketide spiroketals, including hemi-succinates, hemi-fumarates and hemi-furanoates. Reveromycins were identified with the aid of HPLC-DAD-MS and HPLC-DAD-SPE-NMR methodology, and structures were assigned by detailed spectroscopic analysis. The structural and mechanistic requirements for an unprecedented hemi-succinate : ketal-succinyl equilibrium were defined and provided a basis for proposing that reveromycin 4′-methyl esters and 5,6-spiroketals were artifacts. A plausible reveromycin polyketide biosynthesis is proposed, requiring a 2-methylsuccinyl-CoA starter unit, with flexible incorporation of a C6–8polyketide chain extension and diacid esterification units. Structure activity relationship investigations by co-metabolites were used to assess the anticancer, antibacterial and antifungal properties of reveromycins.