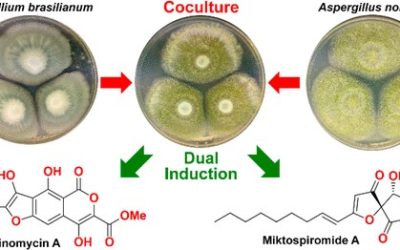
Michael S. Cowled, Daniel Vuong, Andrew Crombie, Ernest Lacey, Peter Karuso and Andrew M. Piggott
Organic & biomolecular chemistry 2021, 18, 5879-5890
Publication Date: July 10, 2020
https://doi.org/10.1039/D0OB01099E
Abstract:
Stereodivergence in Nature encapsulates both enzymatic (biosynthetic) and non-enzymatic (chemical) diversification of natural product scaffolds arising from a single biosynthetic pathway. Herein, we report a fascinating example of stereodivergence for the bacterial polyketide enterocin, which we observed to undergo a series of facile skeletal rearrangements in solution, leading to four distinct isomeric structures. The final distribution of the four isomers was found to be highly sensitive to the conditions used, including solvent, temperature and pH. In this study, we have investigated the kinetics of these isomeric conversions, and using a combination of DFT and thermochemical calculations, were able to establish a mechanism detailing a concerted rearrangement and an unusual “gymnastic” sequence of pseudo-chair-boat conformational interconversions. In addition to these kinetic and mechanistic studies, we also performed a semisynthetic study aimed at stabilising the enterocin scaffold. In total, seven analogues of enterocin were synthesised and investigated for their stability and in vitro activity against a panel of bacteria, fungi, plants and mammalian cells.